NOW APPROVED FOR CKD!
MAKE PROTECTION
YOUR SUPERPOWER
JARDIANCE® protects by reducing risk for
adult patients with CKD*1,2, HF†3,4 and T2D+CVD.‡5

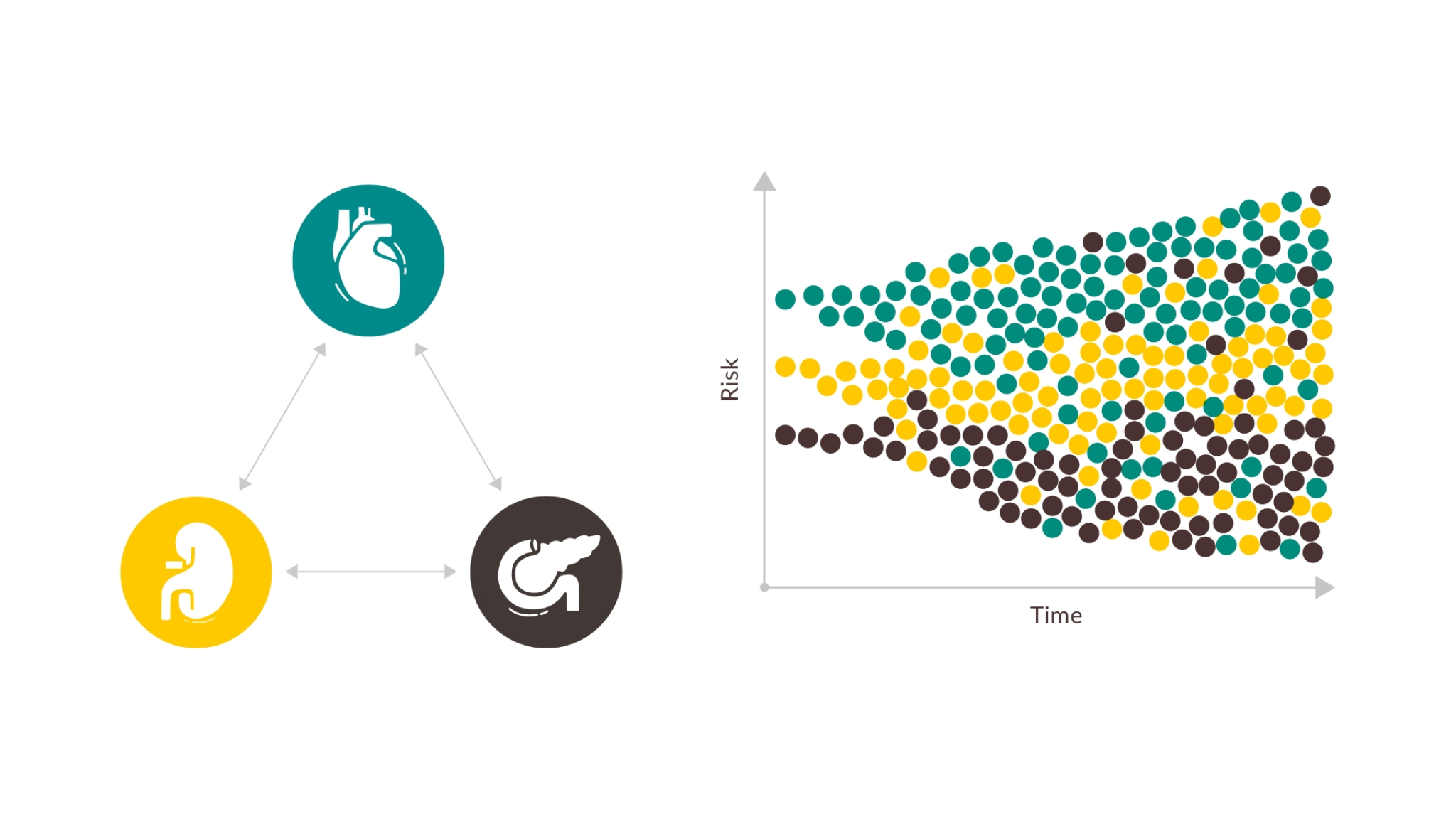
Patients with chronic HF need broad protection by reducing escalating risks6-8

HELP PROTECT YOUR PATIENTS WITH HF - WITH JARDIANCE®
Early intervention is the best way to protect patients from these risks10-14
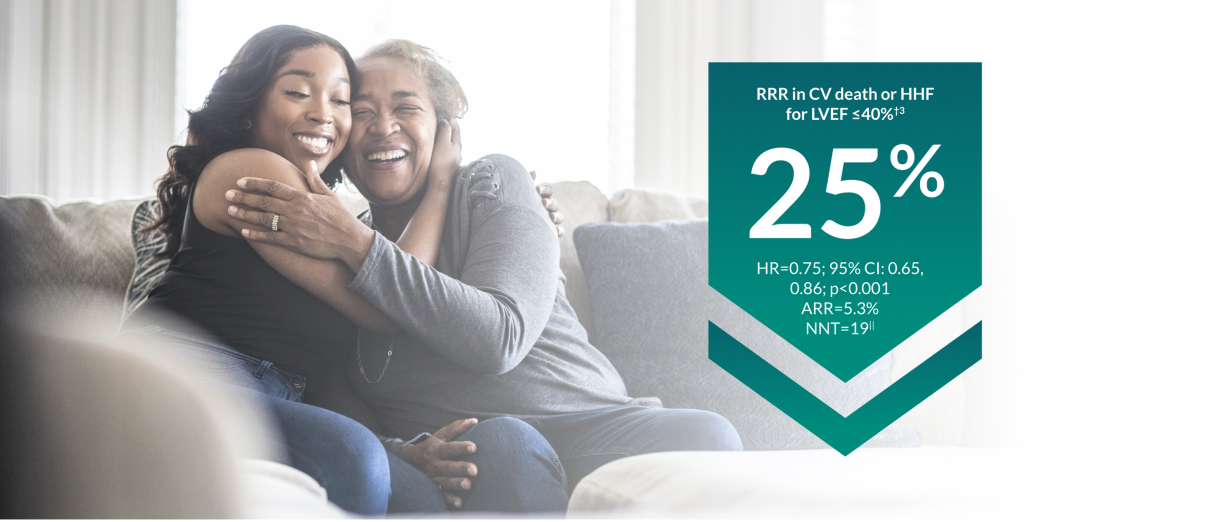
JARDIANCE® protects patients across the LVEF spectrum by reducing the risk of CV death or HHF1,3,4

Build on the proven CV protection of JARDIANCE®
With ~10,000 patients studied in randomized controlled trials 3,4,9, you can rely on the proven CV protection of JARDIANCE®
IN THE TREATMENT OF PATIENTS WITH HF
JARDIANCE® significantly increased KCCQ score across the LVEF spectrum§§15,16
With JARDIANCE®, patients were more likely to experience early and sustained symptom relief§§## when added to background therapy vs placebo15,16

Related Content

GUIDELINE RECOMMENDATIONS

DOSING RECOMMENDATIONS

SAFETY & TOLERABILITY PROFILE
Indication & Footnotes
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
as monotherapy when metformin is considered inappropriate due to intolerance
in addition to other medicinal products for the treatment of diabetes
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.
-
*
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety profile of JARDIANCE® 10 mg (n=3304) was evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease defined as end-stage kidney disease (the initiation of maintenance dialysis or receipt of a kidney transplant), a sustained decrease in the eGFR to <10 ml/min/1.73 m2, a sustained decrease in eGFR of ≥40% from baseline, or death from renal causes. Patients treated with JARDIANCE® experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).2
-
†
In the EMPEROR-Reduced trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3730 patients with HFrEF, the efficacy and safety profile of JARDIANCE® 10 mg (n=1863) was evaluated vs placebo (n=1867). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65, 0.86; p<0.001). In the EMPEROR-Preserved trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety profile of JARDIANCE® 10 mg (n=2997) was evaluated vs placebo (n=2991). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).3,4
-
‡
The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE, composed of death from CV causes, nonfatal MI, or nonfatal stroke, as analyzed in the pooled JARDIANCE® group vs the placebo group. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. The 14% RRR in 3-point MACE (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) was driven by a reduction in the risk of CV death (HR=0.62; 95% CI: 0.49, 0.77).5
-
§
Adult patients with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke.1,5
-
¶
Adult patients with an eGFR ≥ 20, < 45 mL/min/1.73 m2; or an eGFR ≥ 45, < 90 mL/min/1.73 m2 with a uACR ≥ 200 mg/g.2
-
#
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%).3 Adult patients with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%).4
-
||
ARR calculation: JARDIANCE® number of patients with events 361/total number of patients 1863=19.4%; placebo number of patients with events 462/total number of patients 1867=24.7%; 24.7%–19.4%=5.3%. NNT=1/ARR.3
-
**
ARR calculation: JARDIANCE® number of patients with events 415/total number of patients 2997=13.8%; placebo number of patients with events 511/total number of patients 2991=17.1%; 17.1%–13.8%=3.3%.4
-
††
ARR was estimated as the absolute difference in the proportion of events by treatment arm. NNT=1/ARR.4
-
‡‡
Results of prespecified subgroup analyses. EMPEROR-Reduced: Diabetes at baseline (HR=0.72; 95% CI: 0.60, 0.87); no diabetes at baseline (HR=0.78; 95% CI: 0.64, 0.97). eGFR (CKD-EPI) ≥ 60 mL/min/1.73 m2 at baseline (HR=0.67; 95% CI: 0.55, 0.83); eGFR (CKD-EPI) < 60 mL/min/1.73 m2 at baseline (HR=0.83; 95% CI: 0.69, 1.00).2 EMPEROR-Preserved: Diabetes at baseline (HR=0.79; 95% CI: 0.67, 0.94); no diabetes at baseline (HR=0.78; 95% CI: 0.64, 0.95). eGFR (CKD-EPI) ≥ 60 mL/min/1.73 m2 at baseline (HR=0.81; 95% CI: 0.65, 1.00); eGFR (CKD-EPI) < 60 mL/min/1.73 m2 at baseline (HR=0.78; 95% CI: 0.66, 0.91).3,4
-
§§
Change from baseline in clinical summary score (HF symptoms and physical limitations domains) of the KCCQ at Week 52 was a prespecified secondary endpoint in the EMPEROR-Reduced and EMPEROR-Preserved trials. KCCQ change from baseline to 52 weeks. EMPEROR-Reduced: JARDIANCE® 5.8 ± 0.4; placebo 4.1 ± 0.4. EMPEROR-Preserved: JARDIANCE® 4.51, placebo 3.18. Patient-reported outcomes measured changes in KCCQ summary scores. JARDIANCE® led to significant improvements in mean KCCQ-CSS, -TSS, and -OSS, which were apparent as early as 3 months and were sustained at 8 and 12 months. Patients treated with JARDIANCE® were more likely to show clinically meaningful improvements (≥ 5, ≥ 10, and ≥ 15 points) and less likely to experience clinically meaningful deterioration in health status when compared to placebo.3,4,16,17
-
##
Symptoms: shortness of breath, fatigue, or ankle, feet, and/or leg swelling.
ARR=absolute risk reduction; CAD=coronary artery disease; CI=confidence interval; CKD=chronic kidney disease; CKD-EPI=Chronic Kidney Disease Epidemiology Collaboration; CSS=Clinical Summary Score; CV=cardiovascular; CVD=cardiovascular disease; eGFR=estimated glomerular filtration rate; HF=heart failure; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; HR=hazard ratio; KCCQ=Kansas City Cardiomyopathy Questionnaire; LVEF=left ventricular ejection fraction; MACE=major adverse cardiovascular events; MI=myocardial infarction; NNT=number needed to treat; NYHA=New York Heart Association; OSS=Overall Summary Score; PAD=peripheral artery disease; RRR=relative risk reduction; SE=standard error of the mean; TSS=Total Symptom Score; T2D=type 2 diabetes.
References
-
JARDIANCE® [summary of product characteristics]. Ingelheim am Rhein, Germany; Boehringer Ingelheim International GmbH.
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476-2486. doi:10.1016/j.jacc.2017.08.074
-
Sepehrvand N, Savu A, Spertus JA, et al; Alberta HEART Investigators. Change of health-related quality of life over time and its association with patient outcomes in patients with heart failure. J Am Heart Assoc. 2020;9(17):e017278. doi:10.1161/JAHA.120.017278
-
House AA, Wanner C, Sarnak MJ, et al; Conference Participants. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(6):1304-1317. doi.org/10.1016/j.kint.2019.02.022
-
Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568-574. doi:10.1038/s41591-021-01659-1
-
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
-
McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
-
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
-
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Acad Cardiol. 2022;79(17):e263-e421.
-
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(1):1-150.
-
Butler J, Anker SD, Filippatos G, et al; EMPEROR-Reduced Trial Committees and Investigators. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42(13):1203-1212. doi:10.1093/eurheartj/ehaa1007
-
Butler J, Filippatos G, Siddiqi J, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-Preserved trial. Circulation. 2022;145(3):184-193. doi:10.1161/CIRCULATIONAHA.121.057812