OFEV® (nintedanib) | OFEV - Therapy For IPF & SSc-ILD | OFEV
OFEV® nintedanib
OFEV® is a multitargeted tyrosine kinase inhibitor that has been shown to slow interstitial lung disease (ILD) progression across a broad range of patients with pulmonary fibrosis in three large phase 3 clinical trials and an open-label extension study.1–6

OFEV® is indicated in adults for the treatment of idiopathic pulmonary fibrosis (IPF).1
OFEV® is also indicated in adults for the treatment of other chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype.1
OFEV® is indicated in adults for the treatment of systemic sclerosis associated interstitial lung disease (SSc-ILD).1
Adverse events profile
OFEV® has demonstrated a manageable safety and tolerability profile, with 5 years of post-approval clinical evidence in patients with idiopathic pulmonary fibrosis (IPF).4
Read out more about the safety profile of OFEV® in IPF
Additional safety information
OFEV® has an established safety profile with additional safety data on liver enzyme, cardiac events and bleeding events.1,7
Read in detail about the additional safety data for OFEV®
Adverse event management
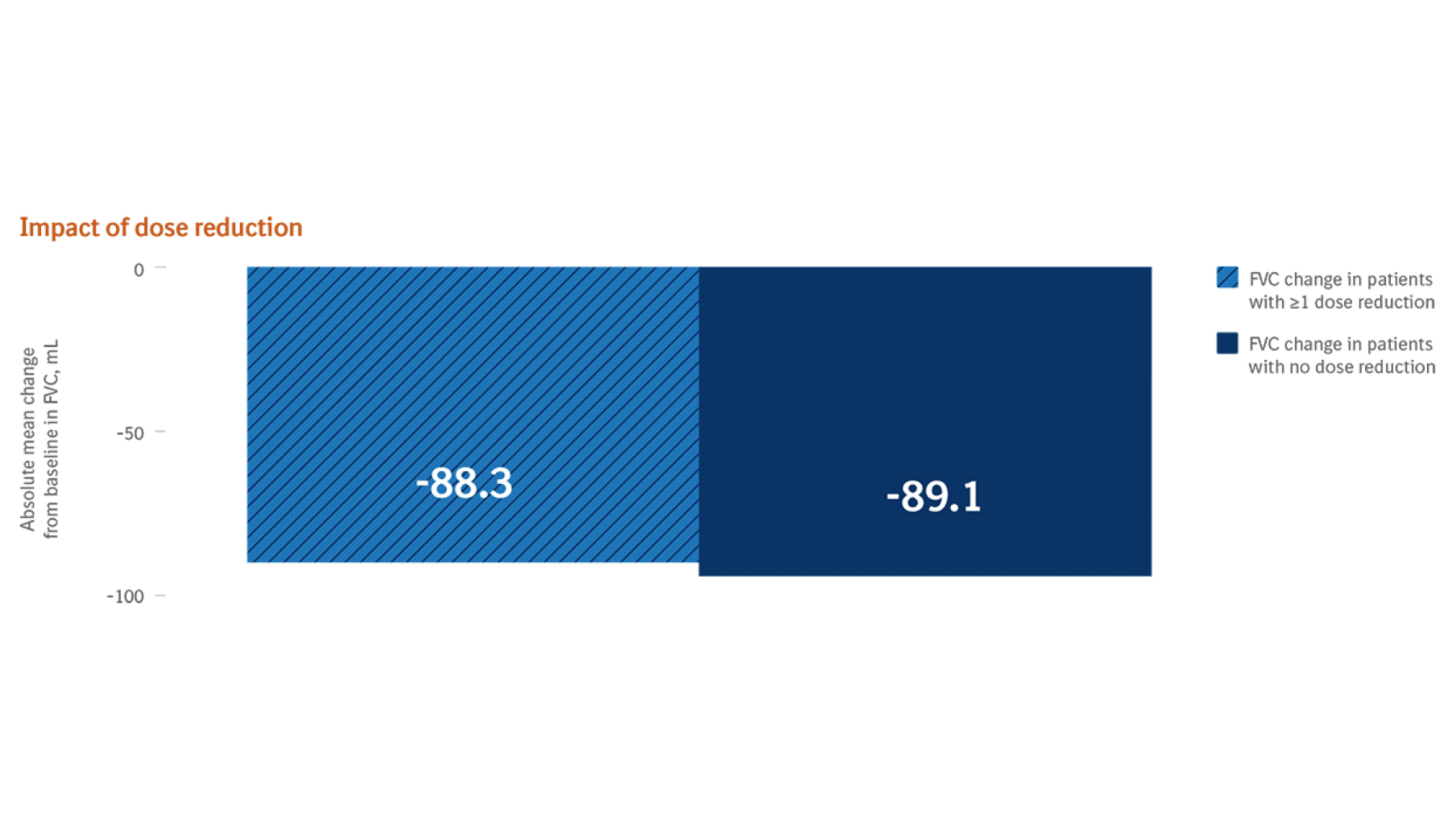
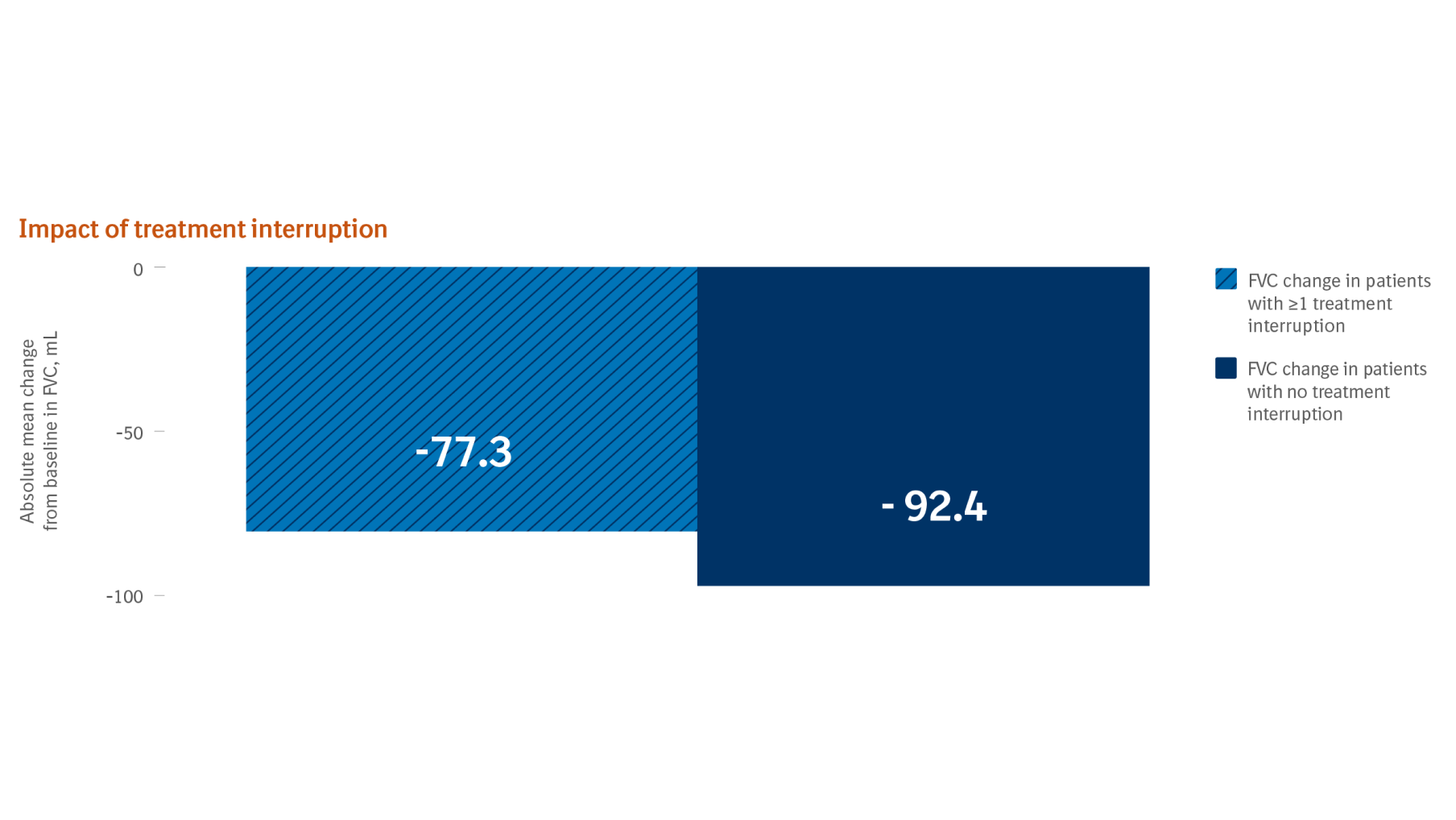
Gastrointestinal (GI)-related adverse events (AEs) can be effectively managed in most patients with dose modification and symptomatic treatment.1
Find out more about managing AEs in your IPF patients
Adverse events profile
OFEV® has demonstrated a manageable safety and tolerability profile, with 5 years of post-approval clinical evidence in patients with idiopathic pulmonary fibrosis (IPF)4
The most common adverse events (AEs) were gastrointestinal (GI)-related and could be effectively managed.1,10
Pooled INPULSIS® and TOMORROW trials10
| Most common AEs* | Placebo (n=508), n (%) | OFEV® (nintedanib) (n=723), n (%) |
|---|---|---|
| Diarrhea | 91 (17.9) | 445 (61.5) |
| Nausea | 36 (7.1) | 176 (24.3) |
| Nasopharyngitis | 79 (15.6) | 93 (12.9) |
| Cough | 75 (14.8) | 93 (12.9) |
| Vomiting | 15 (3.0) | 85 (11.8) |
| Decreased appetite | 24 (4.7) | 81 (11.2) |
| Bronchitis | 56 (11.0) | 76 (10.5) |
| Progression of IPF | 72 (14.2) | 68 (9.4) |
| Upper respiratory tract infection | 55 (10.8) | 65 (9.0) |
| Dyspnea | 59 (11.6) | 55 (7.6) |
- Serious AEs were comparable across treatment groups10
- OFEV® has NOT been associated with photosensitivity3
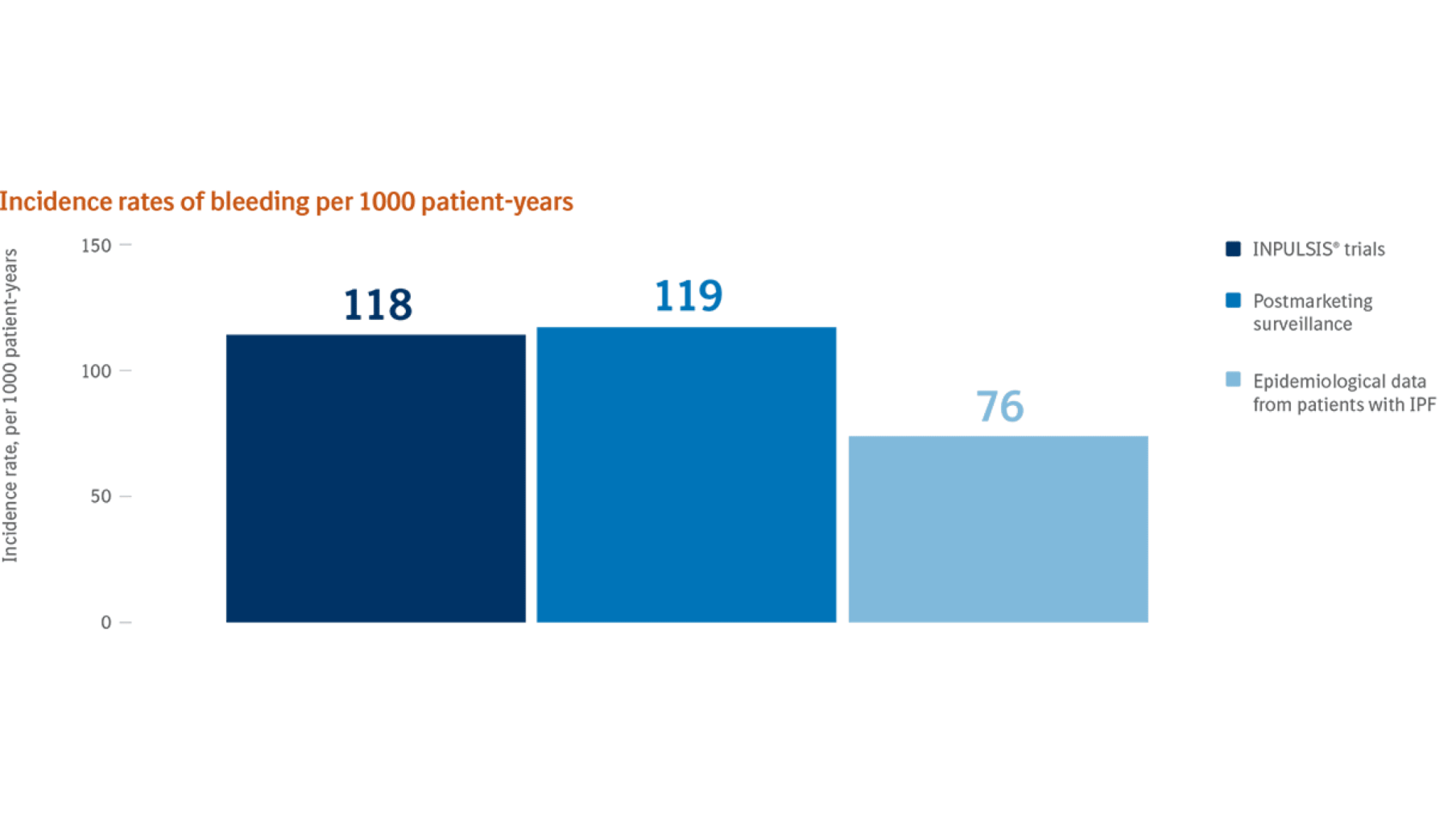
Real-world data
In a postmarketing surveillance study:3,11†

Data were collected over 1 year in a real-world clinical setting

The safety profile of OFEV® was shown to be consistent with INPULSIS® trials

There were no new safety concerns identified
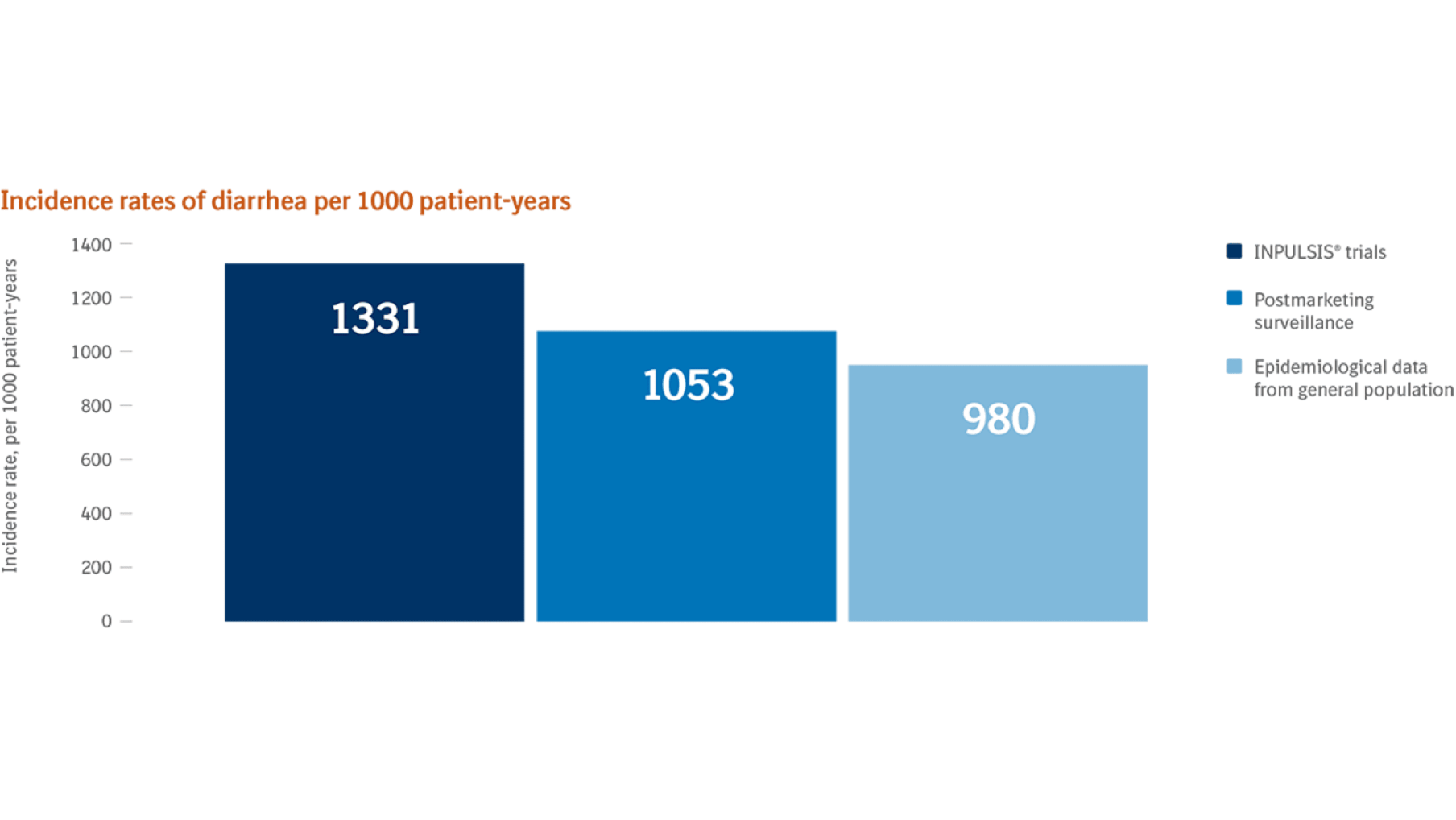
The rate of diarrhea reported in postmarketing surveillance was lower than the rate reported in the INPULSIS® trials and similar to epidemiological data from the general population11
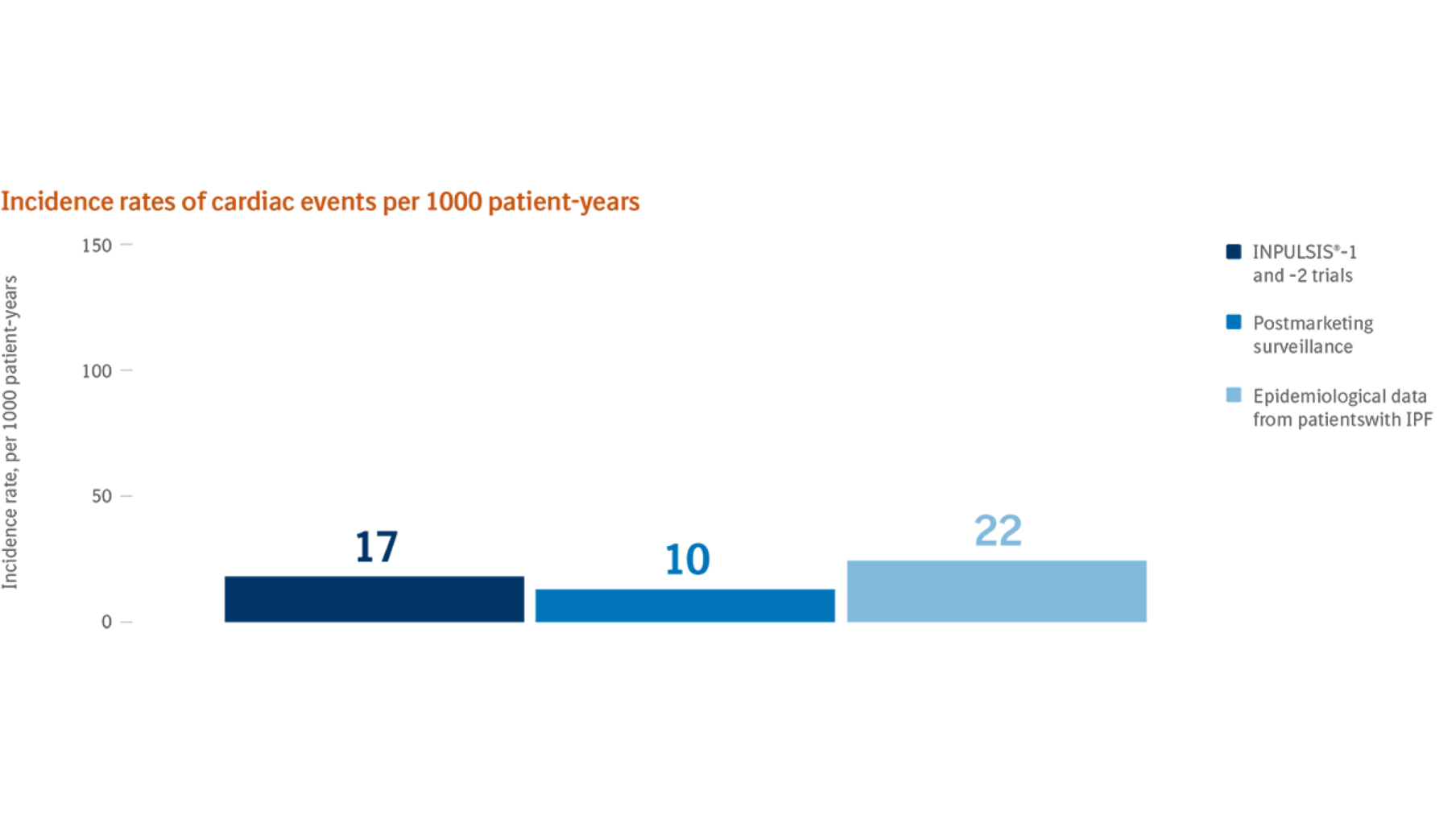
Known risk factors for cardiac events were reported in 42.3% of cases11
The rate of bleeding events‡ reported in postmarketing surveillance was similar to the rate in the INPULSIS® trials, but higher than in epidemiological data from patients with IPF11
Claims-based rates (from epidemiological data) for nonserious bleeding events (e.g. epistaxis) are not accurate as only events that are severe enough for the patient to seek medical attention at a healthcare facility are captured. 11
Long-term exposure
The safety profile of OFEV® observed in the INPULSIS® trials was sustained in the INPULSIS®-ON trial4§
- Continued treatment with OFEV® for up to 68 months had a manageable safety and tolerability profile4¶
- There were no new safety signals identified4
- The safety profile of OFEV® was shown to be consistent with the INPULSIS® trials4‖
Footnotes
- *
The most common AEs were defined as those with an incidence of >10% in either study group.10
- †
The postmarketing surveillance study was based on data collected between the drug’s launch in the United States on 15 October 2014 and a data snapshot on 31 October 2015. Adverse events were coded according to preferred terms in the MedDRA version 18.1. Data have been proactively communicated between specialty pharmacies and patients since the launch of OFEV®.11
- ‡
Bleeding was based on the SMQ “haemorrhage terms (excluding laboratory terms).”11
- §
Per protocol, the off-treatment period between INPULSIS®-1 and -2 and INPULSIS®-ON could be between 4 and 12 weeks.4
- ¶
Median total exposure for those treated with OFEV® in both the INPULSIS® trials and the INPULSIS®-ON trial extension was 44.7 months and the maximum total exposure was 68.3 months.4
- ||
Rates of major adverse cardiovascular events, myocardial infarction, and bleeding in patients were similar to those observed in patients who received placebo during the INPULSIS® trials, suggesting there is no increased risk of these events with long-term use of OFEV®.4
Additional safety information
Liver enzyme elevation
Incidence4
- Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevations ≥3x upper limit of normal (ULN) occurred in approximately 5% of patients and bilirubin elevations ≥1.5x ULN occurred in approximately 2% of patients3
- Transaminase elevations were reversible upon dose reduction or interruption1
Monitoring1
- Conduct liver function tests (hepatic transaminase and bilirubin levels) before treatment initiation, during the first month of treatment, at regular intervals during the subsequent 2 months and periodically thereafter (e.g. at each patient visit or as clinically indicated)
- For AST or ALT elevations ≥3x ULN, dose reduction or interruption of therapy with OFEV® and close monitoring of the patient are recommended
- Once levels return to baseline values, treatment with OFEV® may be resumed at the full dose (150 mg twice daily) or a reduced dose (100 mg twice daily), which subsequently may be increased to the full dose
- If the liver test elevations are associated with clinical signs or symptoms of liver injury (e.g. jaundice), then permanently discontinue therapy with OFEV®
Hepatic function1
- The safety and efficacy of OFEV® have not been studied in patients with moderate or severe hepatic impairment (Child-Pugh B or C). OFEV® is not recommended in these patients
- Patients with mild hepatic impairment (Child-Pugh A) should be treated with a reduced dose (100 mg twice daily) of OFEV®
Pregnancy1
- OFEV® may cause fetal harm in humans. Advise women of childbearing potential to avoid becoming pregnant while receiving treatment with OFEV® and to use highly effective contraception methods during and at least 3 months after the last dose of OFEV®. Advise women using hormonal contraceptives to add a barrier method
- Prior to initiating treatment, conduct a pregnancy test in females of childbearing potential
Cardiac events
- The incidence of serious and nonserious cardiac adverse events (AEs), including ischemic heart disease and cardiac disorders, was balanced between the treatment arms3,7
Summary of cardiac disorder AEs in the INPULSIS® trials3,7
| Placebo (n=423), (%) | OFEV® (n=638), (%) | |
|---|---|---|
| AE cardiac disorders* | 10.6 | 10.0 |
| Serious AE cardiac disorder | 5.4 | 5.0 |
| Fatal AE cardiac disorder | 1.4 | 0.5 |
| Ischemic heart disease† | 4.0 | 4.2 |
| Myocardial infarction | 0.5 | 1.1 |
| Acute myocardial infarction | 0.0 | 0.5 |
| Other ischemic heart disease | 3.1 | 1.7 |
- The incidence rate of myocardial infarction observed for the OFEV®-treated patients remains substantially below the incidence for myocardial infarction in a representative idiopathic pulmonary fibrosis (IPF) population7,12
- The incidence rate of major adverse cardiovascular events (MACE) was similar between both treatment arms in high- and low-cardiovascular (CV) risk patients in a post hoc analysis of pooled INPULSIS®-1 and -2 and TOMORROW data13‡
Bleeding events
- Due to the mechanism of action, OFEV® may be associated with an increased risk of bleeding1
Pooled analysis of INPULSIS®-1 and INPULSIS®-27
| Placebo (n=423), (%) | OFEV® (n=638), (%) | |
|---|---|---|
| Bleeding AEs Epistaxis Contusion |
7.8 0.9 3.1 |
4.1 10.3 1.6 |
| Serious bleeding | 1.4 | 1.3 |
- Nonserious epistaxis and contusion were the most frequently reported bleeding events1,7
- Serious bleeding events occurred with a low and similar frequency in both groups1,7
Note
Patients at known risk for bleeding (including those with inherited predisposition to bleeding or patients receiving a full dose of anticoagulative treatment) were not included in the INPULSIS® trials.1 These patients should only be treated with OFEV® if the anticipated benefit outweighs the potential risk.1
Footnotes
- *
System organ class “cardiac disorder.”12
- †
SMQ “ischaemic heart disease” (includes myocardial infarction).12
- ‡
The category of MACE comprised the following: AEs leading to death from cardiac disorders and vascular disorders; fatal and nonfatal events in the subordinate SMQ “myocardial infarction”; stroke based on selected preferred terms from the subordinate SMQs “haemorrhagic cerebrovascular conditions” and “ischaemic cerebrovascular conditions”; and the preferred terms sudden death, cardiac death, and sudden cardiac death.12
- §
High CV risk was defined as patients who had a history of atherosclerotic CV disease and/or ≥1 CV risk factor at baseline (e.g. smoking history, high blood pressure, or high cholesterol).12
Adverse event management
Gastrointestinal (GI)-related adverse events (AEs) can be effectively managed in most patients with dose modification and symptomatic treatment1
Management of GI side effects should begin as early as possible after onset of symptoms1
1. Supportive medications1
- Antidiarrheals, such as loperamide
- Antiemetic therapy, such as a dopamine receptor antagonist or an H1 antihistaminic
2. Dose adjustment1
- Temporary treatment interruption or dose reduction (100 mg twice daily) should be considered if symptomatic treatment is ineffective*
- If a patient does not tolerate 100 mg twice daily, treatment with OFEV® should be discontinued
3. Dietary changes
- Adequate hydration at first sign of diarrhea1
- Diet of bland, low-fiber foods, such as white bread, bananas, eggs, cooked potatoes without the skin, and fish, chicken, or turkey without the skin14
Footnotes
- *
In addition to symptomatic treatment if applicable, the management of adverse reactions to OFEV® could include dose reduction and temporary interruption until the specific adverse reaction has resolved to levels that allow continuation of therapy. OFEV® treatment may be resumed at the full dose (150 mg twice daily) or a reduced dose (100 mg twice daily). If a patient does not tolerate 100 mg twice daily, treatment with OFEV® should be discontinued. In case of interruptions due to AST or ALT elevations >3x ULN, once transaminases have returned to baseline values, treatment with OFEV® may be reintroduced at a reduced dose (100 mg twice daily), which subsequently may be increased to the full dose (150 mg twice daily).1
For more detailed information, please visit the website of your specific country and follow your local SmPC:

Denmark
Visit websiteAbbreviations
-
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTD-ILD, connective tissue disease-associated interstitial lung disease; CV, cardiovascular; FVC, forced vital capacity; GI, gastrointestinal; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MACE, major adverse cardiovascular events; MedDRA, Medical Dictionary for Regulatory Activities; SMQ, standardised Medical Dictionary for Regulatory Activities Queries; SSc-ILD, systemic sclerosis-associated interstitial lung disease; ULN, upper limit of normal.